Fukushima Project
Measuring radiation in Fukushima
Attended radiation training program held by Research Center for Nuclear Physics, Osaka University
The program aims for a deeper understanding of radiation. I attended as a member through crowdfunding.
The program includes fieldwork in Fukushima where the nuclear disaster happened. There, we visited the still contaminated restricted sites and were able to hear a lot of information from local residents. We learned that people (even people in Japan) avoid buying food grown in Fukushima even when the radiation level is tested and shown to be safe. It is causing the food in Fukushima which used to be top delicious in Japan (eg. rice) to diminish or be sold as cheap ones.


We took some blueberry grown in Fukushima from a farmer we visited and measured the radiation level. After the analysis of the measurement, we made a poster presenting the results we got and compared it with multiple food radiation standards in the world in a way easy to understand for the symposium of the Fukushima program. For the result of the analysis, the radiation measurement of the blueberry sample showed no difference from background radiation. We also made blueberry jam from the blueberry we harvested for the audience to try out in order to call for rational choice on products made in Fukushima.



Report will be available soon.
Some basic knowledge for understanding the measurement:
radiation basics
Alpha Particles: Come from the decay of the heaviest radioactive elements (eg. uranium, radium and polonium). They are so heavy that they use up their energy over short distances and are unable to travel very far from the atom, thus not being able to penetrate the outer layer of skin. However, they can be very dangerous inside the body if inhaled or get into the body through a cut.
Beta Particles: They are more penetrating and less damaging than alpha particles. They travel farther in air but can be stopped by a layer of clothing or by a thin layer of a substance such as aluminum. Some beta particles are capable of penetrating the skin but are most hazardous when they are inhaled or swallowed.
Gamma Rays: They are weightless packets of energy, unlike alpha and beta particles which have both energy and mass. They are similar to visible light but have much higher energy. Often emitted along with alpha or beta particles during radioactive decay. Gamma rays are a radiation hazard for the entire body. They can easily penetrate barriers that can stop alpha and beta particles, such as skin and clothing. Several inches of a dense material like lead, or even a few feet of concrete may be required to stop them. As gamma rays pass through the body, they can cause ionizations that damage tissue and DNA.
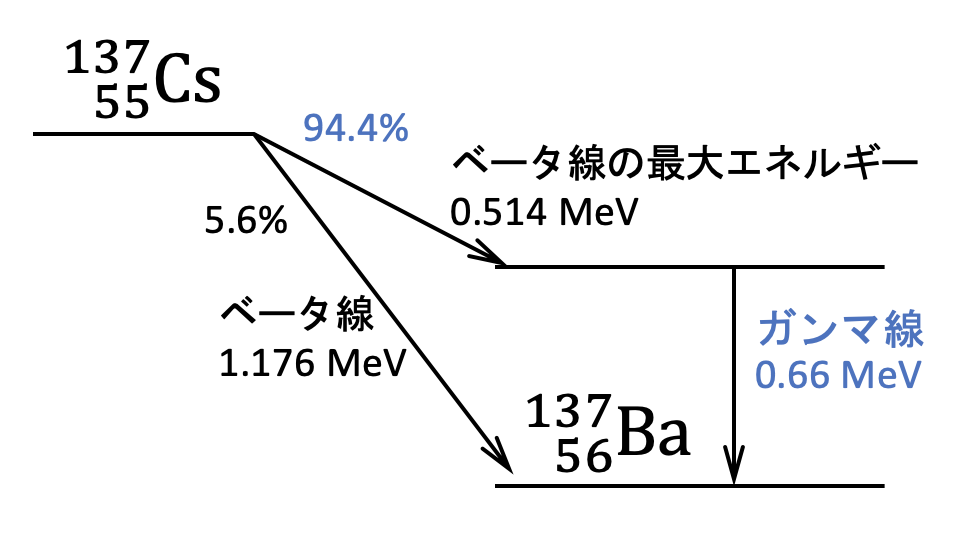
Cesium-137 emits a beta particle and a gamma-ray per nucleus by 94% chance when they decay. The most common technique to distinguish Cs-137 is by analyzing the energy spectrum of gamma rays since Cs-137 is known to emit gamma rays with the energy of 662 kilo-electronvolt (keV).
We did measurement with NaI detector during the program in Fukushima, and with Ge detector after we came back to Osaka.